++ 50 ++ 30‘ã ”¯Œ^ ƒƒ“ƒO ƒXƒgƒŒ[ƒg 206255
Graph g(x)=(x3)^3 Find the point at Tap for more steps Replace the variable with in the expression Simplify the result Tap for more steps Subtract from Raise to the power of The final answer is Convert to decimal Find the point at Tap for more stepsWritten in one line, it looks like this 640 g O 2 x (1 mol O 2 / g O 2) x (4 mol NO / 5 mol O 2) x ( g NO / 1 mol NO) = 480 g NO 3) Written in individual steps, the solution looks like this (a) convert grams of O 2 to moles 640 g / g/mol = mol O 2 (b) use a ratio and proportion involving O 2 and NOPlugging in g = 98 and q = 30 degrees a x = 49 m/s 2 Plug this, and x o = 0, v ox = 0, and x = 3 m, into the equation x x o = v ox t ½ a x t 2 gives 3 = 245 t 2 solving for t tells us that the box takes 111 s to slide down the ramp

Vapor X5 Next Gen Pre Workout Powder Explosive Energy Supplement Blue Raspberry 30 Servings 9 6oz Walmart Com
30'ã "¯Œ^ ƒƒ"ƒO ƒXƒgƒŒ[ƒg
30'ã "¯Œ^ ƒƒ"ƒO ƒXƒgƒŒ[ƒg-Mol O 1600 g O = 333 mol C = 666 mol H = 333 mol O CH 2O empirical formula mass of CH 2O molar mass of lactate 9008 g 3003 g 3 C 3H 6O 3 is the molecular formula O 333 333 H 666 333 C 333 333 Combustion apparatus for determining formulas of organic compounds Figure 35 C nH m (n ) O 2 = n CO(g) H 2O(g) m 2 m 2 differenceA gigagram is a unit of mass in the Metric System The symbol for gigagram is Gg There are gigagrams in a gram




Variation Of A Normalized Shear Modulus G G Max And B Damping Download Scientific Diagram
1 g = oz To convert 30 grams into ounces we have to multiply 30 by the conversion factor in order to get the mass amount from grams to ounces We can also form a simple proportion to calculate the result 1 g → oz 30 g → M (oz) Solve the above proportion to obtain the mass M in ouncesConversion in the opposite direction The inverse of the conversion factor is that 1 gram is equal to e05 times 30 kilograms It can also be expressed as 30 kilograms is equal to 1 e05 grams grams O 2 = 90 grams H 2 O x (1 mol H 2 O/18 g) x (1 mol O 2 /2 mol H 2 O) x (32 g O 2 /1 mol H 2) grams O 2 = (90 x 1/18 x 1/2 x 32) grams O 2 grams O 2 = 80 grams O 2 To produce 90 grams of water, 10 grams of hydrogen gas and 80 grams of oxygen gas are needed
Calculate the equilibrium concentration for each species from the initial concentrations and the changes H 2 = Br 2 = 0010 x = 0010 0008 = 0002 M for each HBr = 2x = 2(0008) = 0016 M Check your answer by substituting the equilibrium concentrations into the equilibrium expression and see if the result is the same as the equilibrium constantGraph g(x)=(x3) Rewrite the function as an equation Remove parentheses Use the slopeintercept form to find the slope and yintercept Tap for more steps The slopeintercept form is , where is the slope and is the yintercept Find the values of and using the form301 Grams (g) = Ounces (oz) Visit 301 Ounces to Grams Conversion Grams The gram (SI unit symbol g) is a metric system unit of mass It is equal to one onethousandth of the SI base unit, the kilogram, or 1 kg Today, the gram is the most widely used unit of measurement for nonliquid ingredients in cooking and grocery shopping
113k Followers, 118 Following, 80 Posts See Instagram photos and videos from OG (@og30)Number of entities as there are atoms in 12 g of carbon12 • Exactly 12 g of carbon12 contains 6022 x 10 23 atoms • One mole of H 2 O molecules contains 6022 x 10 23 molecules • 1 mole contains 6022 x 10 23 entities (Avogadro's number) Concept 1Where X is the result in oz and Y is the amount of g we want to convert 30 Grams to Ounces Conversion breakdown and explanation 30 g to oz conversion result above is displayed in three different forms as a decimal (which could be rounded), in scientific notation (scientific form, standard index form or standard form in the United Kingdom




Pacon Spectra Deluxe Bleeding Art Tissue 12 X 18 5 Color Ble Rietkoetter Cosmeticals De



Apps Dtic Mil
1 mg = (1/1000) g = 0001 g The mass m in grams (g) is equal to the mass m in milligrams (mg) divided by 1000 m(g) = m(mg) / 1000(the limit of a sum is the sum of the limits) 2lim x!af(x) g(x301 g ≅ 1062 oz An alternative is also that one ounce is approximately zero point nine four two times thirty point one grams Conversion table grams to ounces chart For quick reference purposes, below is the conversion table you can use to convert from grams to ounces grams (g) ounces (oz) 311 grams 1097 ounces




Given Vectors A 51 4and 7 21 5j Find 4ă Chegg Com
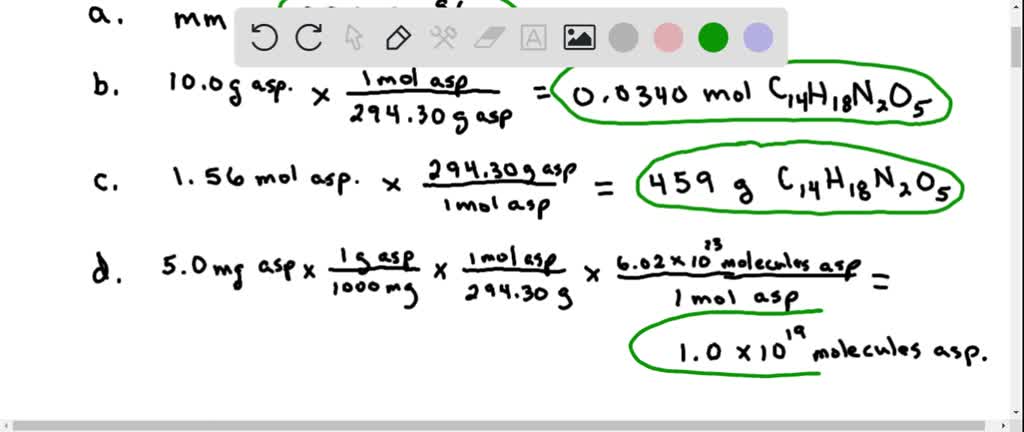



Solved Aspartame Is An Artificial Sweetener That Is 160 Times Sweeter Than Sucrose Table Sugar When Dissolved In Water It Is Marketed As Nutra Sweet The Molecular Formula Of Aspartame Is C14h18n2o5 A Calculate The
Mass of a compound of a solution calculation Mass (g) = Concentration (mol/L) x Volume (L) x Molecular Weight (g/mol)If 35 g of X reacts with 105 g of Y, what is the percent by mass of X in the compound that is formed ?When we first got introduced a function composition we looked at of actually evaluating functions at a point or compositions of functions at a point what I want to do in this video is come up with expressions that define a function composition so for example I want to figure out what is f of G of X f of G of X and I encourage you to pause the video and try to think about it on your own well G



Jencam De



30mm Round
Conversion Table For quick reference purposes, below is a conversion table that you can use to convert from g to kg Grams to Kilograms Conversion Chart grams (g) kilograms (kg) 1 g 0001 kg 2 g(414 g O/114 g) x 100 = 363% O with compounds, find molar mass of the compound and molar mass of the element you are trying to find and times by the subscript for that element within the formula, divide molar mass of element by total molar mass of compound and multiply by 100 to findMolar mass of K = 391 g Molar mass of Mn = 549 g Molar mass of O = 160 g Molar mass of KMnO4 = 391 g 549 g (160 g x 4) Molar mass of KMnO4 = 1580 g What other calculations you can do with the molarity calculator?



2




Vapor X5 Next Gen Pre Workout Powder Explosive Energy Supplement Blue Raspberry 30 Servings 9 6oz Walmart Com
1 mole C2H6, 6 moles of H g x 6 g = 3007 g/mole Or, 3007 g in 1 mole C2H6 So, we say the molar mass of ethane is 3007 g/mol (that's grams per mole) 1 mole Na2Cr2O7, 2 mole of Na 229 g x 2 g 1 mole Na2Cr2O7, 2 mole of Cr g x 2 g 1 mole Na2Cr2O7, 7 moles of O g x 7 gWhat is a gram (g)?EECS 3 Homework–5Solutions Total Points 40 Page 69 56) Suppose that ƒis an invertible function from Y to Z and g is an invertible function from X to Y Show that the inverse of the composition f o g is




Metabolic Endotoxemia Initiates Obesity And Insulin Resistance Diabetes




Ioj4pb91bc3m
G(x)= x2 1 x (8) f(x) = 3x 4 (9) f( ) = 3( ) 4 (10) f(g(x)) = 3(g(x)) 4 (11) f(x2 1 x) = 3(x2 1 x) 4 (12) f(x 2 1 x) = 3x 3 x 4 (13) Thus, (f g)(x) = f(g(x)) = 3x2 3 x 4 Let's try one more composition but this time with 3 functions It'll be exactly the same but with one extra step Find (f g h)(x) given f, g, and hKg to Grams How to convert Grams to Kilograms 1 gram (g) is equal to 0001 kilograms (kg) 1 g = (1/1000) kg = 0001 kg The mass m in kilograms (kg) is equal to the mass m in grams (g) divided by 1000 m (kg) = m (g) / 1000 Example Convert 5 g to kilograms3 x 2 mol H 2O x 1802 g H 2O 8006 g NH 4NO 3 1 mol NH 4NO 3 1 mol H 2O Step 6 Check that all units cancel out except the unknown unit at the end;



Page 2 Cirrus L High Resolution Stock Photography And Images Alamy



Chart Visualization Pandas 1 3 3 Documentation
Dennis was born in Washington DC to Barbara F Milburn, NJ and Joseph BJ Herrity Philadelphia, PA on He was a second child and a brother to Joseph Dennis grew up the first seven years of his life in Washington DC and then moved with the familyBigO Notation BigO notation is used to express the time complexity of an algorithm W h i i h We can assume that any operation requires the same amount of time The time complexity of an al orithm can be The time complexity of an algorithm can5 Given a Rate Law, How much will rate change with change in concentration The reaction CHCl 3(g) Cl 2(g) → CCl 4(g) HCl(g) has the following rate law Rate = kCHCl 3Cl 2If the concentration of CHCl 3 is increased by a factor of five while the concentration of Cl 2 is kept the same, the rate will a double



Analog Of Electromagnetically Induced Transparency In An E Shaped All Dielectric Metasurface Based On Toroidal Dipolar Response




Area Of A Regular Hexagon Video Khan Academy
Maj László 'Szatyi' Szatmári renewed his qualification on JAS39 Gripen Eight years later of his first centrifuge training (see video on this channel) he iThe gravitational force equivalent, or, more commonly, gforce, is a measurement of the type of force per unit mass – typically acceleration – that causes a perception of weight, with a gforce of 1 g (not gram in mass measurement) equal to the conventional value of gravitational acceleration on Earth, g, of about 98 m/s 2 Since gforces indirectly produce weight, any gforce can be30 g to tablespoon = 2 tablespoon 40 g to tablespoon = tablespoon 50 g to tablespoon = tablespoon 100 g to tablespoon = tablespoon 0 g to tablespoon = tablespoon ›› Want other units?
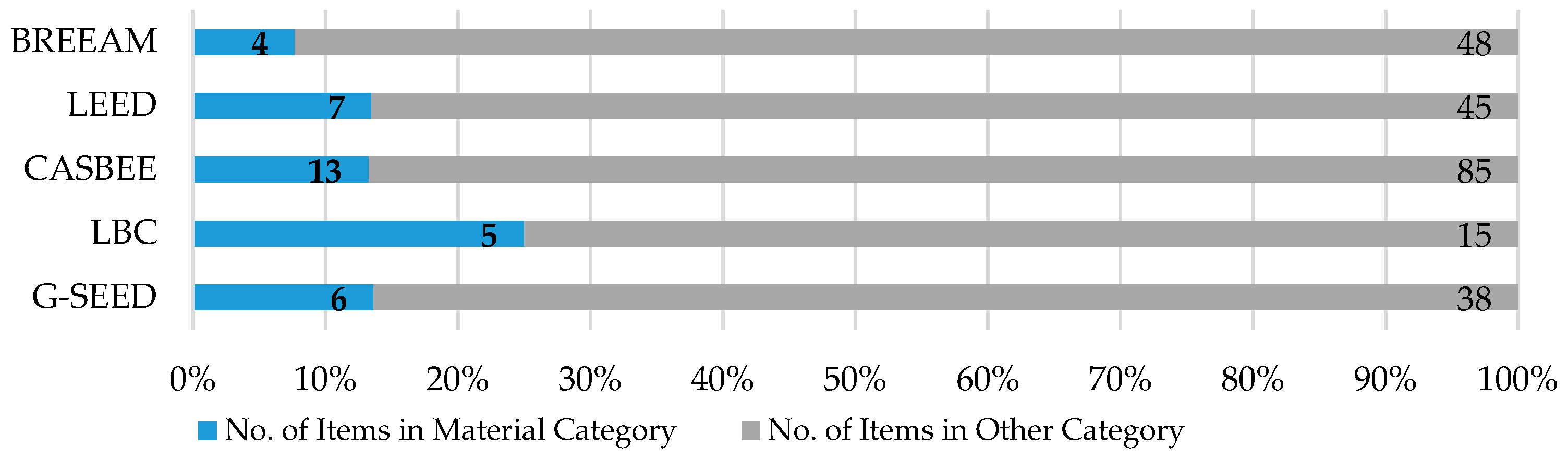



Sustainability Free Full Text Critical Review Of The Material Criteria Of Building Sustainability Assessment Tools Html



2
The amount of purine = the amount of pyramidine Rule 2 The amount of AT ≠ amount of GC This ratio varies among different organisms but same in different tissues of the same organismBig O notation is useful when analyzing algorithms for efficiency For example, the time (or the number of steps) it takes to complete a problem of size n might be found to be T(n) = 4n 2 − 2n 2As n grows large, the n 2 term will come to dominate, so that all other terms can be neglected—for instance when n = 500, the term 4n 2 is 1000 times as large as the 2n termSolve your math problems using our free math solver with stepbystep solutions Our math solver supports basic math, prealgebra, algebra, trigonometry, calculus and more




Oxifast Mr Lornoxicam And Thiocolchicoside Tablets 8mg 8mg West Coast Pharmaceuticals




Bidrag Till Ka Nnedom Av Finlands Natur Och Folk Natural History 119 R2 To S I A A O Am O Ia A S Cd M A Bo Co P
Practice problem 22 % X = 35 g X 140 g compound x 100% = 25 % X 35 g X 105 g Y = 140 g compound % Y = 105 g Y 140 g compound x 100% = 75 % YModels G and GB Spun Aluminum • Downblast Centrifugal Roof Exhaust Fans Greenheck models G and GB centrifugal roof exhaust fans provide the industry's best performance and durability for general clean air applications where air is discharged downward, toward the roof surface • Broadest performance in the industry, up toYou can do the reverse unit conversion from tablespoon to g, or enter any two units below
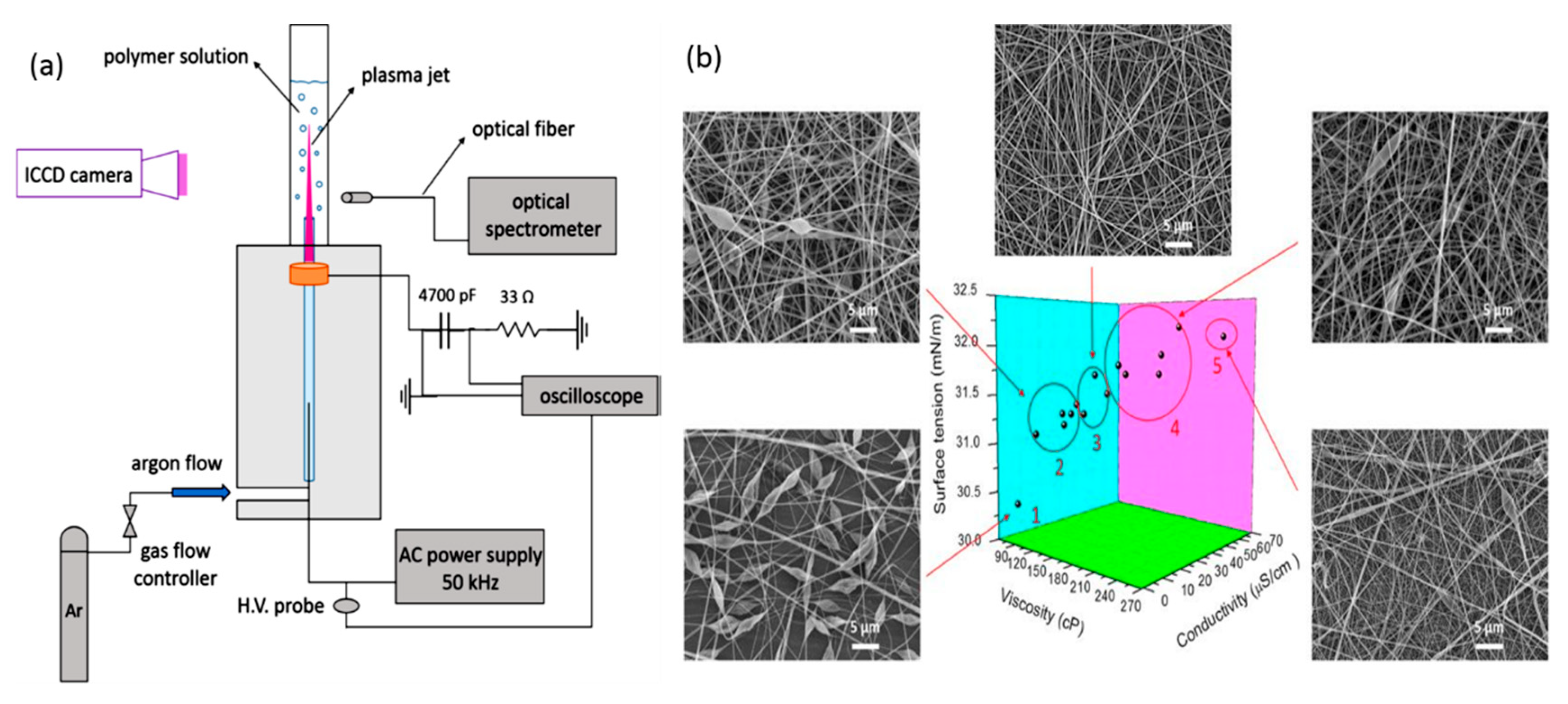



Materials Free Full Text Applications Of Plasma Liquid Systems A Review Html




Bivariate Diagram Of The Lower Third Molar M 3 Of Giraffa Stillei Download Scientific Diagram
The following rules apply to any functions f(x) and g(x) and also apply to left and right sided limits Suppose that cis a constant and the limits lim x!a f(x) and lim x!a g(x) exist (meaning they are nite numbers) Then 1lim x!af(x) g(x) = lim x!af(x) lim x!ag(x) ;30 Grams (g) = Milligrams (mg) Visit 30 Milligrams to Grams Conversion Grams The gram (SI unit symbol g) is a metric system unit of mass It is equal to one onethousandth of the SI base unit, the kilogram, or 1 kg Today, the gram is the most widely used unit of measurement for nonliquid ingredients in cooking and grocery shoppingAn online gof fog calculator to find the (fog) (x) and (gof) (x) for the given functions In this online fog x and gof x calculator enter the f (x) and g (x) and submit to know the fog gof function Fog and Gof are the function composites or the composite functions f o g means Fcomposeg of x written as (f o g) (x) or f (g (x)), and G o f




Aem 30 0300 X Series Wideband Gauge Afr O2 Air Fuel Ratio 8 0 1 1 2 1 16 Ebay




Evaluating Composite Functions Video Khan Academy
3 1055 The metabolic oxidation of glucose, C 6H 12O 6, in our bodies produces CO 2, which is expelled from our lungs as a gas C 6H 12O 6 (aq) O 2 (g) 6 CO 2 (g) 6 H 2O (l) Calculate the volume of dry CO 2 produced at body temperature (37 °C) and 0970 atm when 245 g of glucose is consumed in this reactionSearch the world's information, including webpages, images, videos and more Google has many special features to help you find exactly what you're looking forX = g (this is the molr mass of the hydrate since one mole of it was present at the start) 4) Determine mass, then moles of water − 1059 = g g / g = 699 5) The formula Na 2 CO 3 7H 2 O Solution #2 1) Assume 100 g of Na 2 CO 3 nH 2 O is present Therefore, of the 100 grams Na 2 CO 3 = 457



2
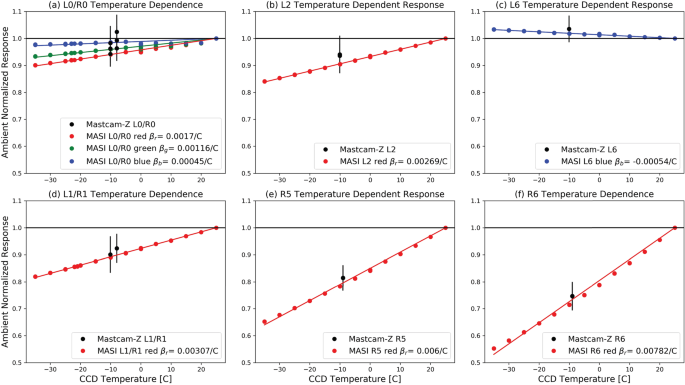



Pre Flight Calibration Of The Mars Rover Mastcam Zoom Mastcam Z Multispectral Stereoscopic Imager Springerlink
Free math lessons and math homework help from basic math to algebra, geometry and beyond Students, teachers, parents, and everyone can find solutions to their math problems instantly 12 g iron / 100 g sample = x g iron / 250 g sample Crossmultiply and divide x= (12 x 250) / 100 = 30 grams of iron How to Calculate Volume Percent Concentration of a Solution Volume percent is the volume of solute per volume of solution This unit is used when mixing together volumes of two solutions to prepare a new solutionTitle show_temppl Author connorja Created Date AM




My Publications Bughz O Keena In Hindi Page 30 31 Created With Publitas Com




Term Complications And Subsequent Risk Of Preterm Birth Registry Based Study The Bmj
A gram is a unit of mass in the Metric System The symbol for gram is g There are 1,000,000,000 grams in a gigagram What is a gigagram (Gg)?Multiply across numerators and divide by denominators molar mass to mole mole to mole ratio mole to molar mass 250 g NH 4NO 3 x 1 mol NH 4NO 3 x 2 mol H 2O x 1802 g H 2O = 113 g H 2O 8006 g NHSearch the world's information, including webpages, images, videos and more Google has many special features to help you find exactly what you're looking for




In Host Mathematical Modelling Of Covid 19 In Humans Abstract Europe Pmc



2



2
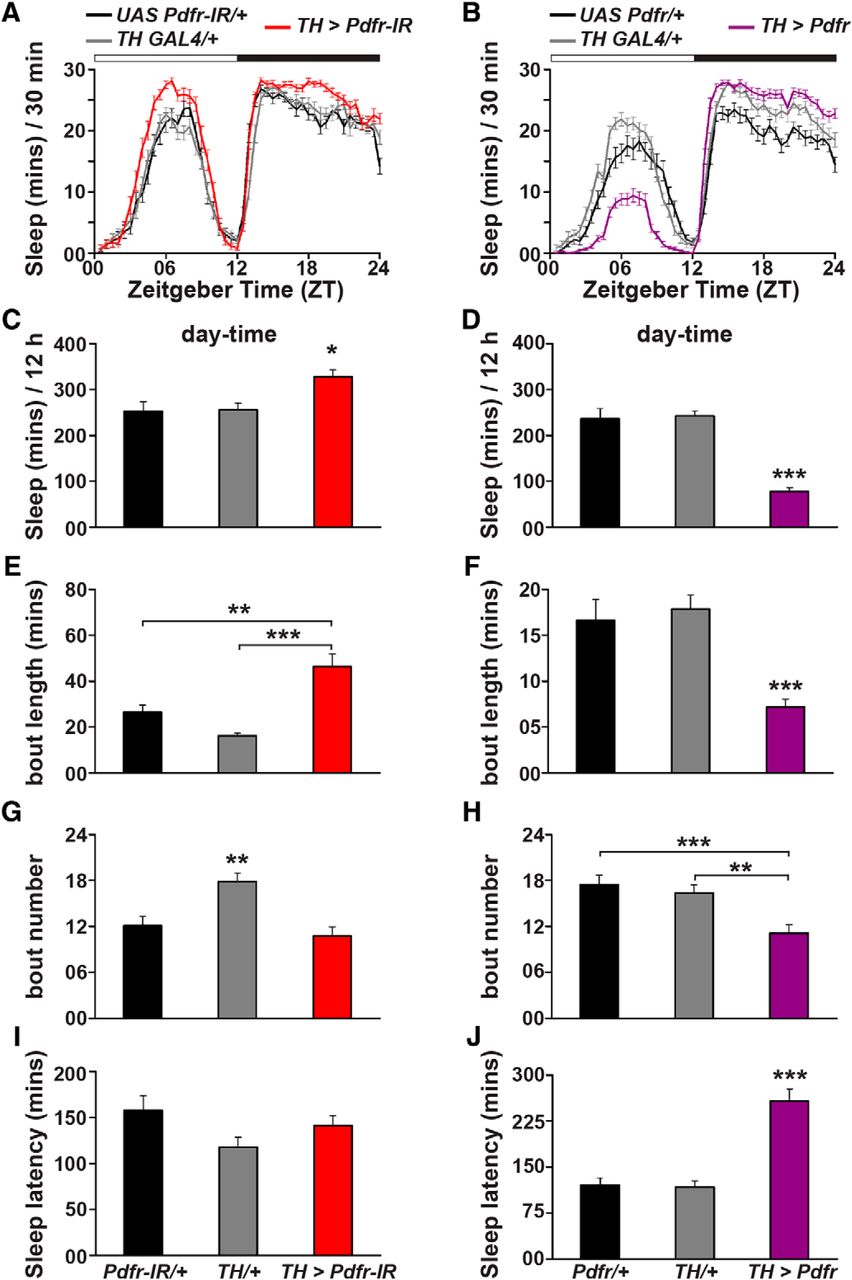



Wakefulness Is Promoted During Day Time By Pdfr Signalling To Dopaminergic Neurons In Drosophila Melanogaster Eneuro




Modeling And Predicting Total Hydrogen Adsorption In Nanoporous Carbon Materials For Advanced Nuclear Systems Sciencedirect



Page 2 O 5a High Resolution Stock Photography And Images Alamy
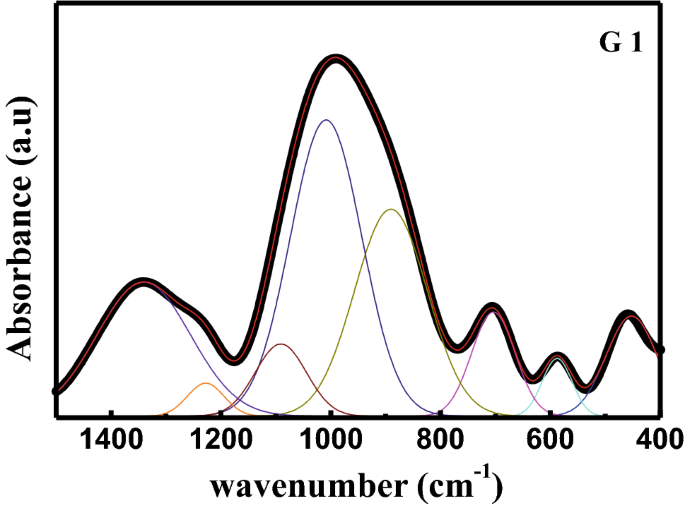



Investigation Of Structural And Radiation Shielding Properties Of 40b2o3 30pbo 30 X Bao X Zno Glass System Springerlink
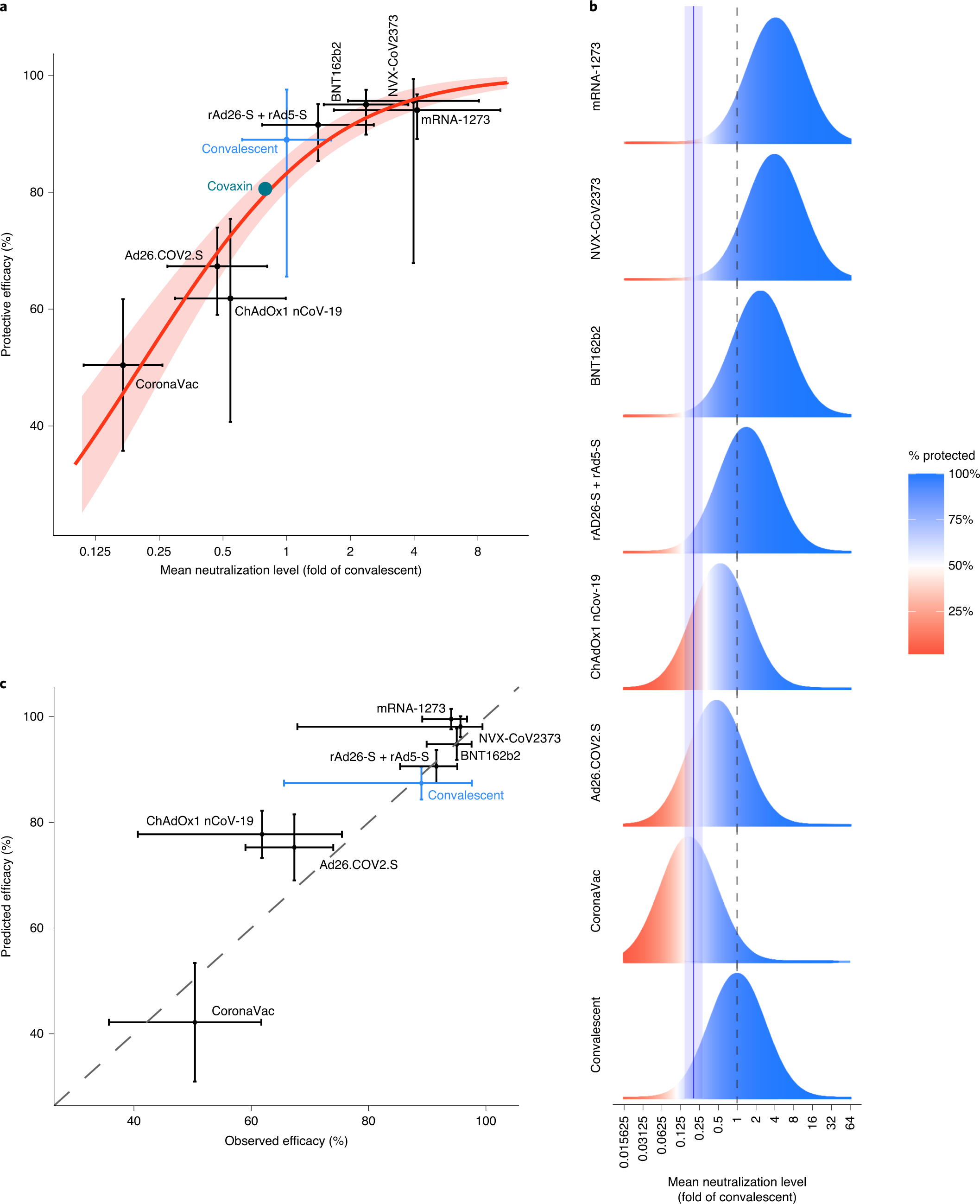



Neutralizing Antibody Levels Are Highly Predictive Of Immune Protection From Symptomatic Sars Cov 2 Infection Nature Medicine




Premium Kitchen Towels 16a A A X 26a A A Phoenix Mall Large Pack 6 A A A



2



2



Aip Scitation Org



Math Umd Edu



2
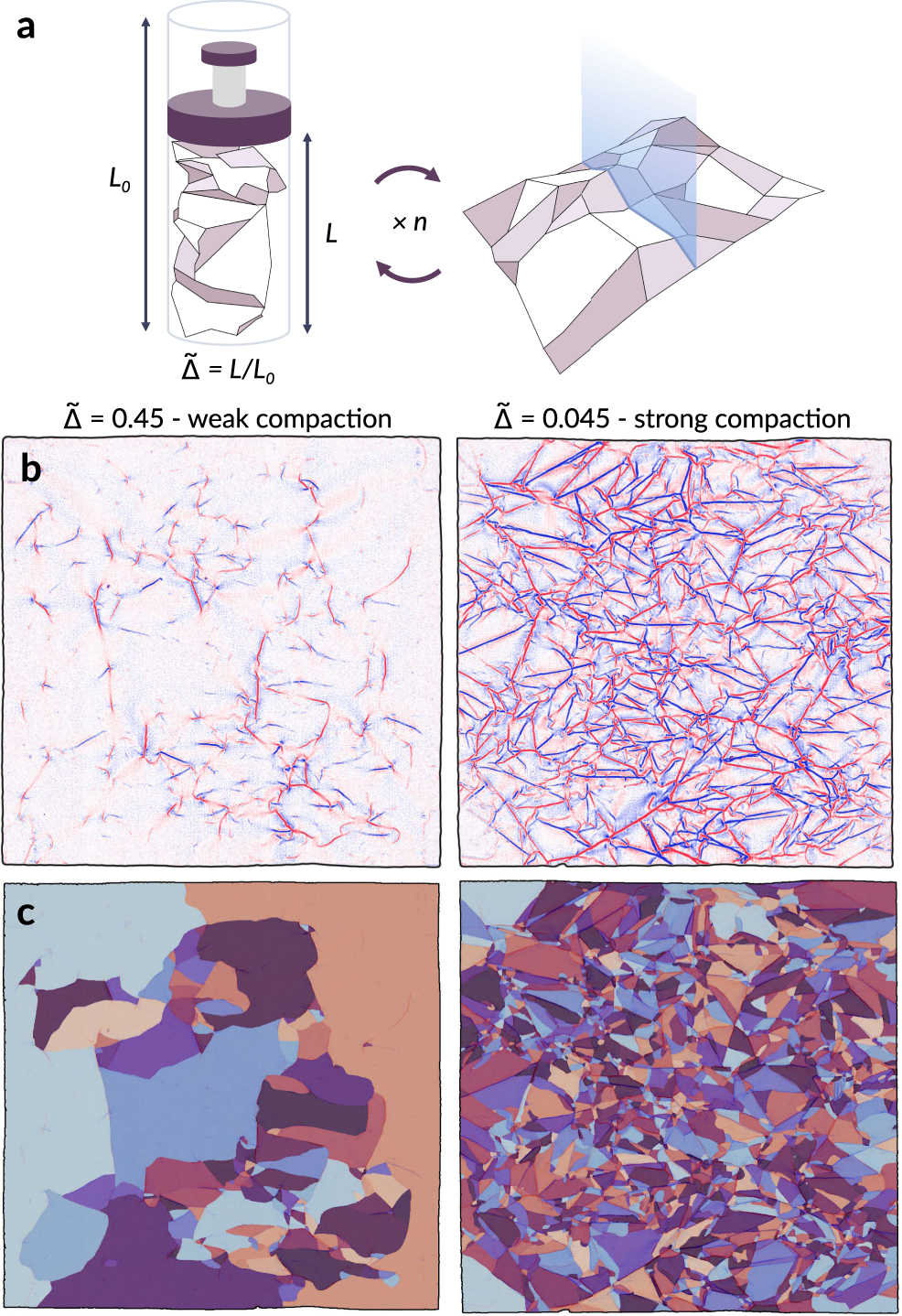



A Model For The Fragmentation Kinetics Of Crumpled Thin Sheets Nature Communications




Consider The Following Economy C 0 85 Y T Ca Ca 600 25 R T 450 0 225 Y Ip 1500 30 R G 1900 Nx 950 0 0625 Y A What Are The Values Of The Autonomous Net Export Nxa And Homeworklib




Cannot Start Outlook In Cached Mode Outlook Microsoft Docs




Index Of Wp Content Uploads Wplms Assignments Folder 4550



2



2



2




Monolithic Perovskite Tandem Solar Cells A Review Of The Present Status And Advanced Characterization Methods Toward 30 Efficiency Jost Advanced Energy Materials Wiley Online Library




30 Handy Bash Shell Aliases For Linux Unix Macos Nixcraft




An Outbreak Of Thyrotoxicosis Caused By The Consumption Of Bovine Thyroid Gland In Ground Beef Nejm




Shape Evolution Of Highly Deformed 75 Kr And Projected Shell Model Description Yang Yingchun Shanghai Jiao Tong University Shanghai August 24 Ppt Download




Vector A Has A Magnitude Of 10 Units And Makes An Angle Of 30 With The Positive X Axis Vector B Has A Magnitude Of Units And Makes And Angle Of 30




Reglamento De Comprobantes De Venta Retenci N Y Documentos Complemen




Page Middle Homepage



Degruyter Com



2
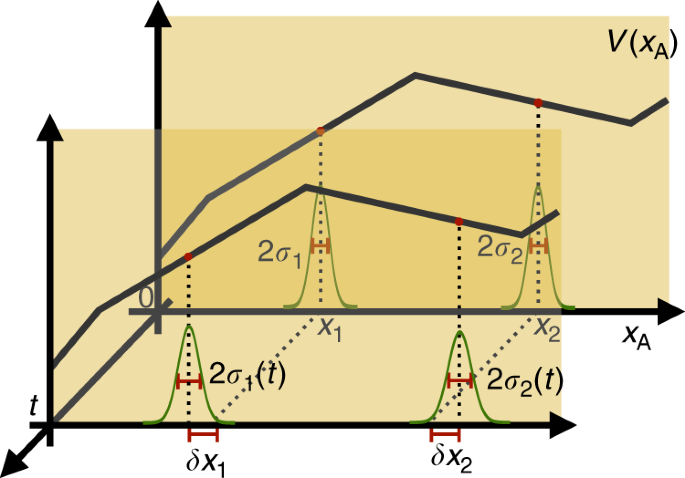



Quantum Mechanics And The Covariance Of Physical Laws In Quantum Reference Frames Nature Communications
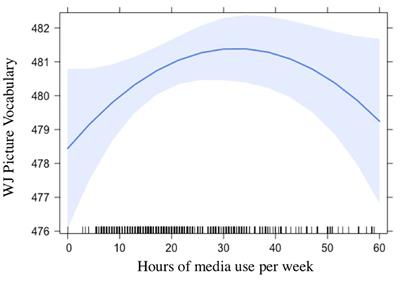



Frontiers Characteristics Of Children S Media Use And Gains In Language And Literacy Skills Psychology



Vector A Has A Magnitude Of 8 00 Units And Makes An Angle Of 45 0 With The Positive X Axis Vector B Also Has A Magnitude Of 8 00 Units And Is Directed Along The
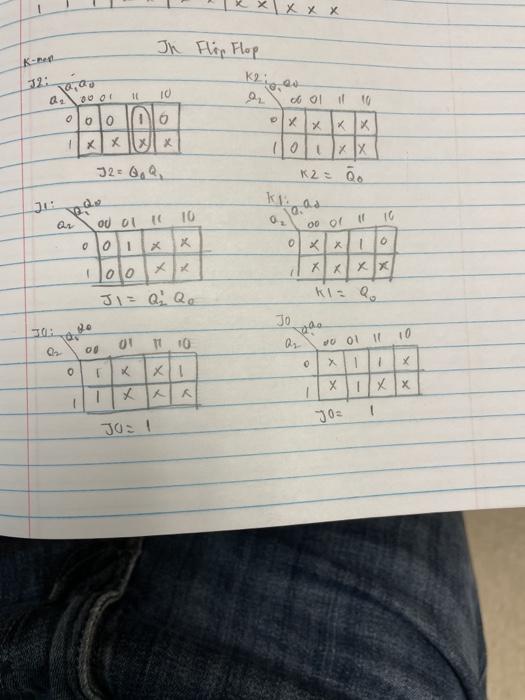



1 X X X Jh Flip Flop K 12 10 00 11 10 70 Col 110 0 Chegg Com




In Depth Analysis Of Laboratory Parameters Reveals The Interplay Between Sex Age And Systemic Inflammation In Individuals With Covid 19 Sciencedirect
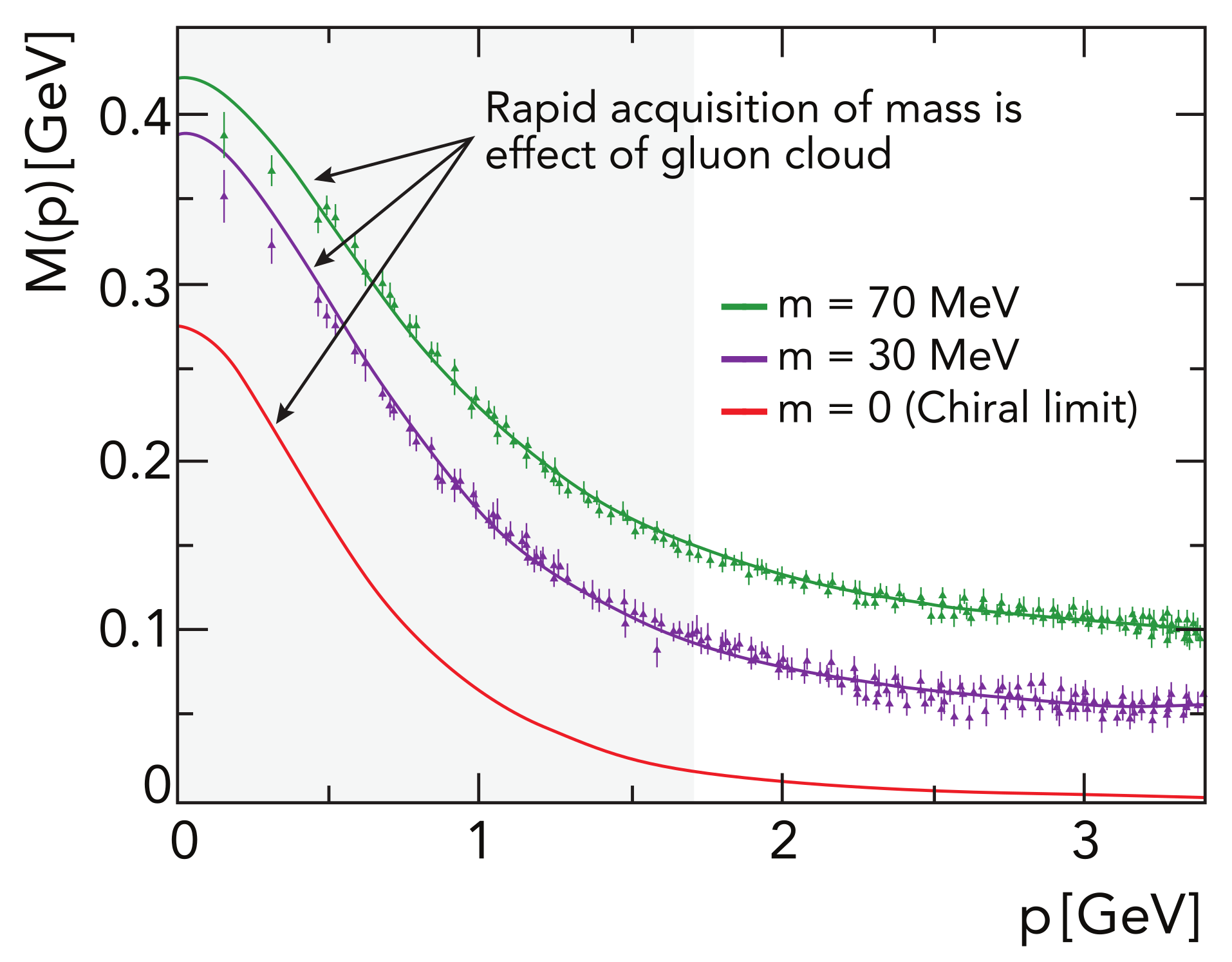



Symmetry Free Full Text Empirical Consequences Of Emergent Mass Html




A Hierarchical Bayesian Perspective On Majorization Minimization For Non Convex Sparse Regression Application To M Eeg Source Imaging Iopscience




Amazon Com Purina Fortiflora Probiotics For Dogs Pro Plan Veterinary Supplements Powder Probiotic Dog Supplement 30 Ct Box Pet Probiotic Nutritional Supplements Pet Supplies



2




Binder Jet 3d Printing Process Parameters Materials Properties Modeling And Challenges Sciencedirect



2




Sequence Of The Periodic Points X 0 X 1 X R 1 In The Fibers Download Scientific Diagram




Variation Of A Normalized Shear Modulus G G Max And B Damping Download Scientific Diagram




Regulating Plant Physiology With Organic Electronics Pnas




Modeling And Predicting Total Hydrogen Adsorption In Nanoporous Carbon Materials For Advanced Nuclear Systems Sciencedirect




Learned Sparcom Unfolded Deep Super Resolution Microscopy Biorxiv



2




Prediction Of Biogas Production From Chemically Treated Co Digested Agricultural Waste Using Artificial Neural Network Sciencedirect




Variation Of Dt X 2 T G Th Vs X Mol Of Pbo Content In A D90 2 Xthsb 2 Download Scientific Diagram
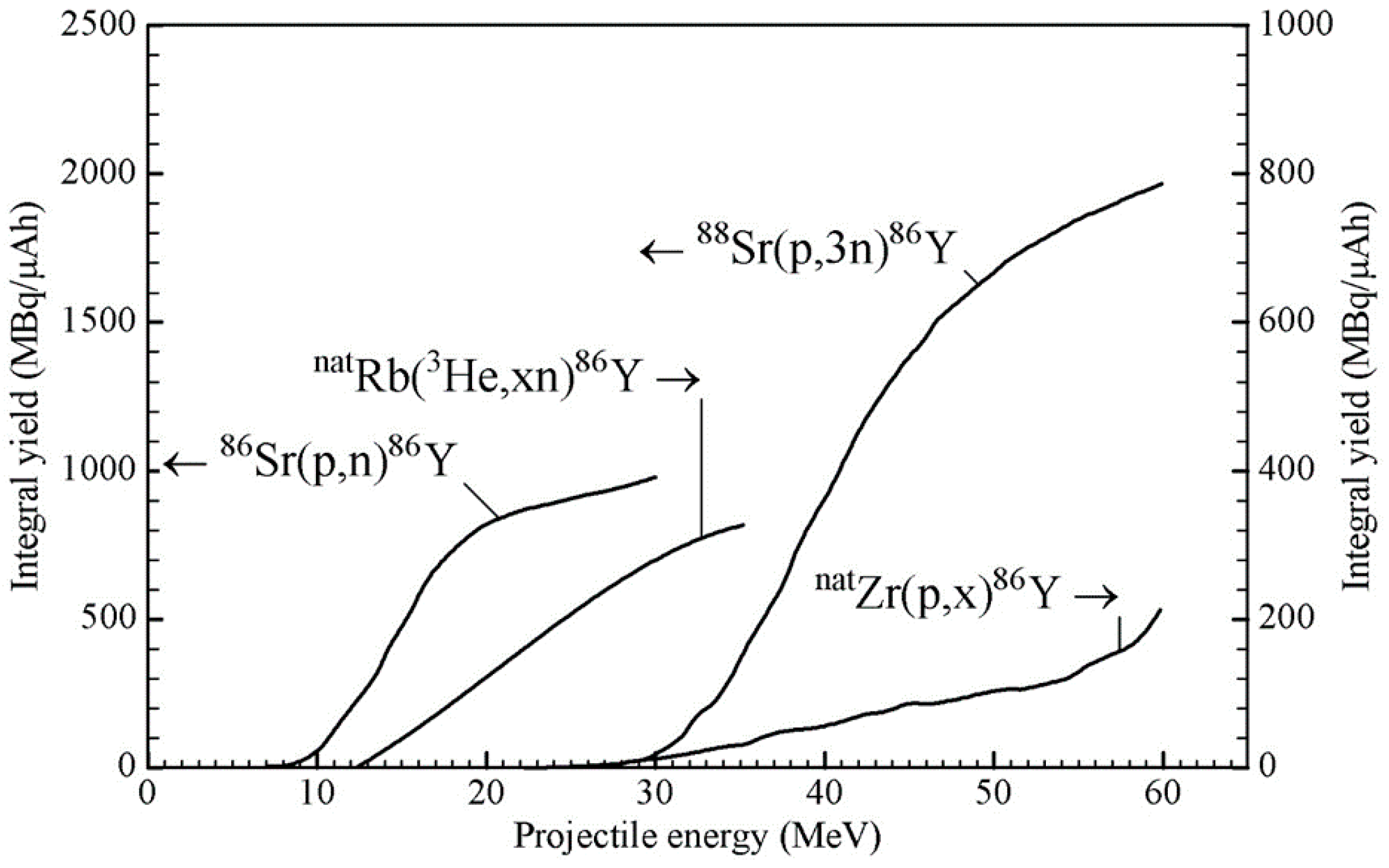



Pharmaceuticals Free Full Text The Beginning And Development Of The Theranostic Approach In Nuclear Medicine As Exemplified By The Radionuclide Pair 86y And 90y Html
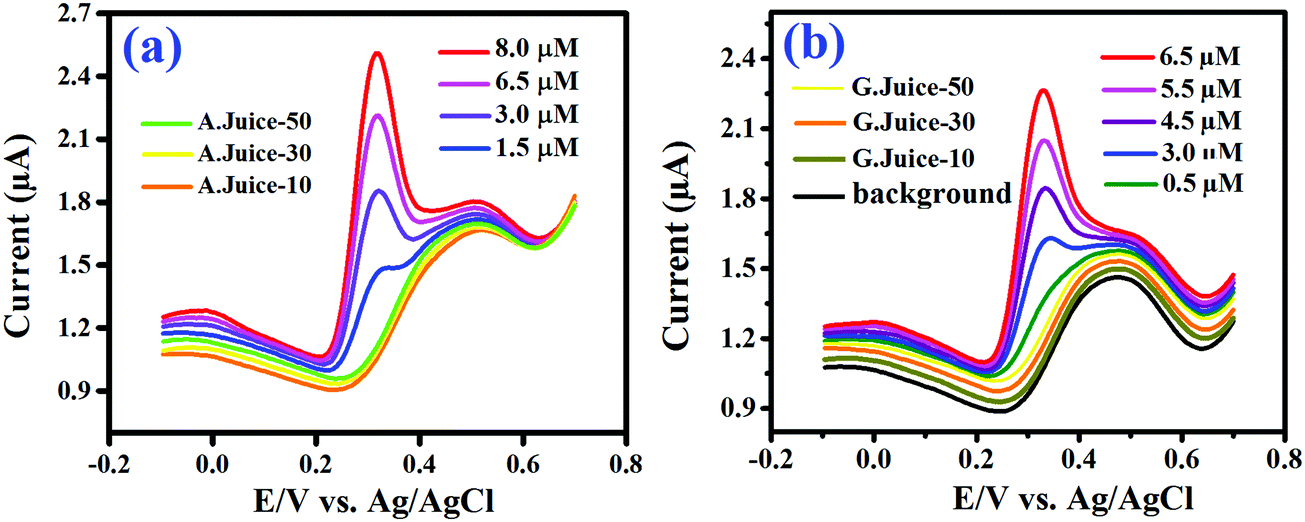



Sr Doped Nio 3 Nanorods Synthesized By A Simple Sonochemical Method As Excellent Materials For Voltammetric Determination Of Quercetin New Journal Of Chemistry Rsc Publishing Doi 10 1039 C9njb
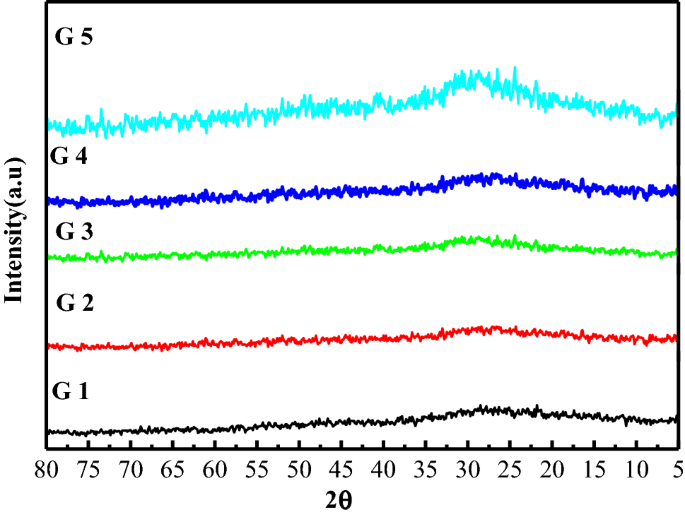



Investigation Of Structural And Radiation Shielding Properties Of 40b2o3 30pbo 30 X Bao X Zno Glass System Springerlink




Lipton Black Iced Tea Mix Decaffeinated Unsweetened 30 Qt Walmart Com




Fomerrey Photos Facebook



1




In Depth Analysis Of Laboratory Parameters Reveals The Interplay Between Sex Age And Systemic Inflammation In Individuals With Covid 19 Sciencedirect



2



2



Arxiv Org



Alt Codes List Alt Key Codes Symbols Sheet Unicode Character Table
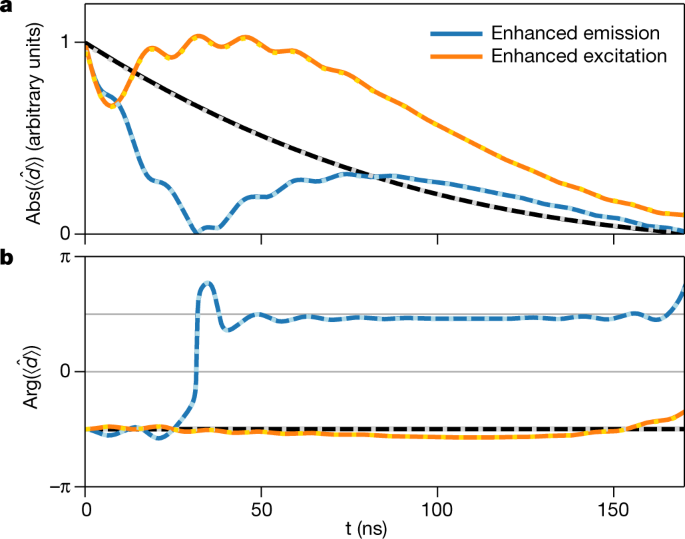



Coherent X Ray Optical Control Of Nuclear Excitons Nature
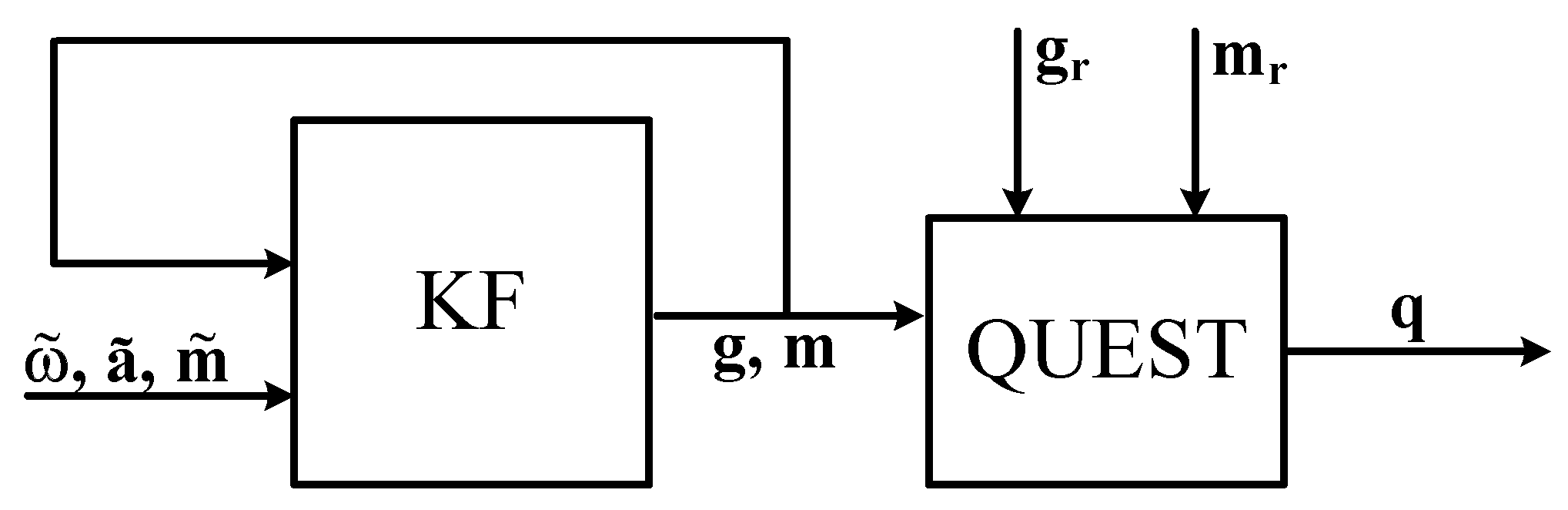



Sensors Free Full Text Survey Of Motion Tracking Methods Based On Inertial Sensors A Focus On Upper Limb Human Motion Html




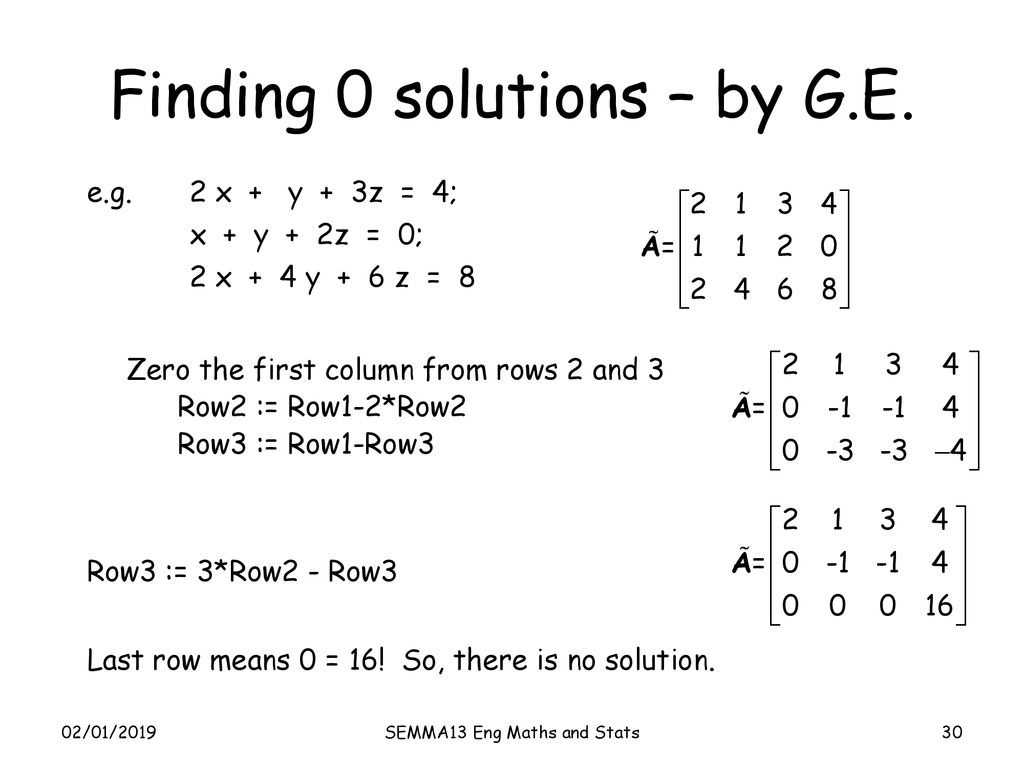



Vector And Matrix Material Ppt Download




Solved Two Vectors A And B Have Equal Magnitudes Of 12 Units These Vectors Are Making Angles 30 And 1 With X Axis Respectively Their Sum Is R Find The



Wcd Dominant Patterns Of Interaction Between The Tropics And Mid Latitudes In Boreal Summer Causal Relationships And The Role Of Timescales




4 30 Points Harmonic Oscillator With Perturbation Recall The Hamiltonian Of An Harmonic Oscillator In 1d Homeworklib




Dr Harrison Zold Photos Facebook




Ignacio Manuel Altamirano Photos Facebook



Ieeexplore Ieee Org



30




Adala C Kok A Mada Rvonula S Kutata Sa Hoz A Fa Sti Fecske 18 Bevi Magyarorsza Gi Nagy Tavaszi Megfigyela C Se Alapja N 1 D Co 00 00 11 Lt Ft Lt A A A L 1a C B Ctl T



30




1 Cseas Kiw Go Faj Ij Mqfjs Ne Kiu V C Z Fis Ts Js Khzt Feilna J D A Ifia Tsu Js Khzt Fe Ecaªjidia Ts Js ம ணவர கள ன தன னம ப க க ய வளர த தல Ppt Download




Hk 4 6 30mm Wikipedia




Monolithic Perovskite Tandem Solar Cells A Review Of The Present Status And Advanced Characterization Methods Toward 30 Efficiency Jost Advanced Energy Materials Wiley Online Library
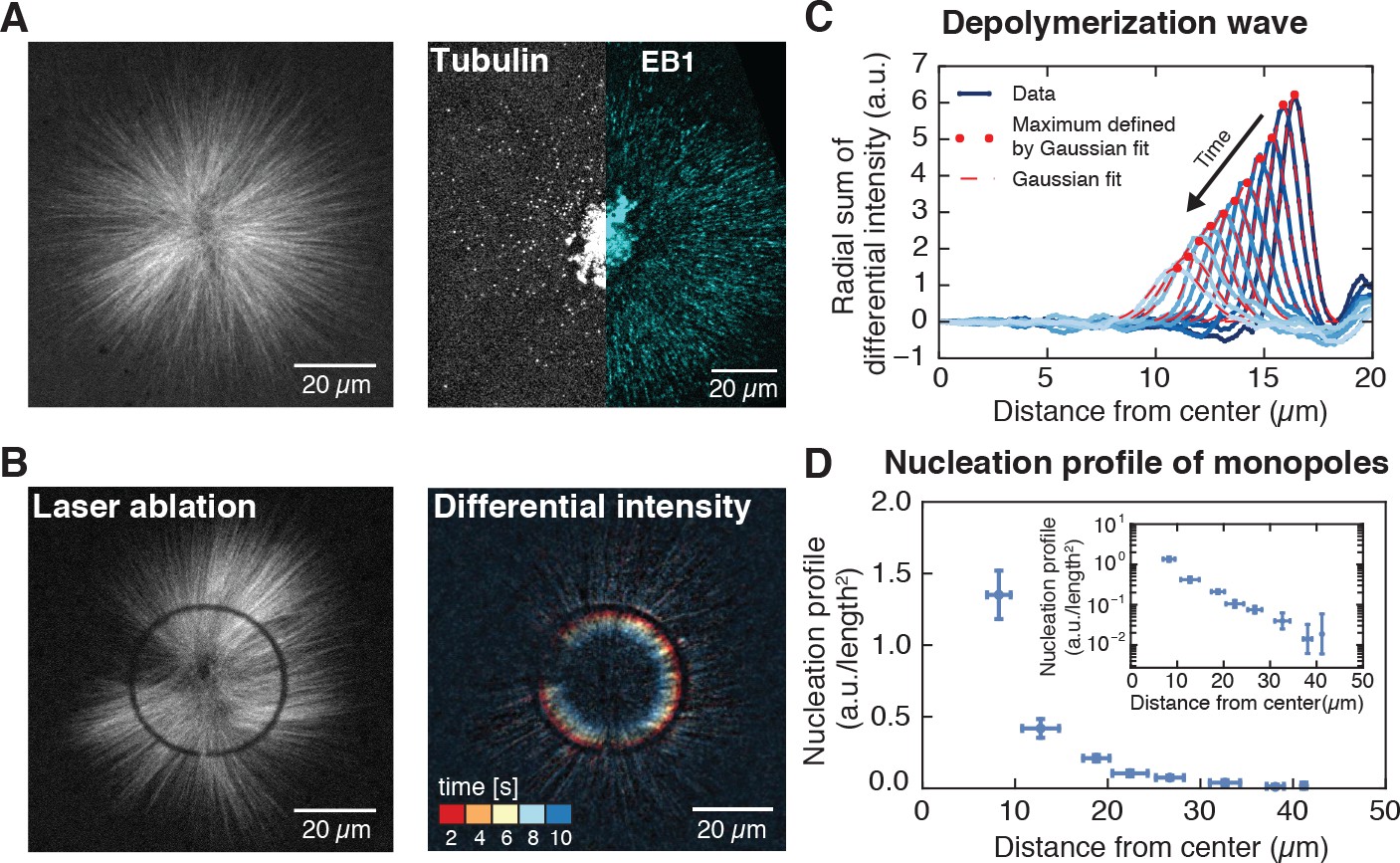



Autocatalytic Microtubule Nucleation Determines The Size And Mass Of Xenopus Laevis Egg Extract Spindles Elife



Journals Sagepub Com
コメント
コメントを投稿